Different Light Responses
- There was an observed difference in the light responses of the optic nerve fibers and the caudal photoreceptor
- These results make sense due to the different functions of the each nerve fiber
- Optic nerve fibers function in regards to in image-forming light responses (e.g. Identifying the location of predators)
- The caudal photoreceptor functions in regards to non-image forming light responses, (e.g. circadian rhythms)
- Therefore, it makes sense that the optic nerve fibers have faster firing rates because image forming light responses must be processed quickly, in order to have adaptive value. Non-image forming light responses, however, do not.
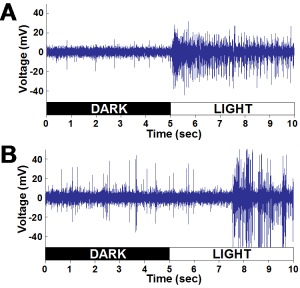
- The difference in the speed of each light response can be seen in Fig. 11.
- Fig. 11 indicates that the caudal photoreceptor possesses a much longer latency of response, compared to the optic nerve fibers
- It is important to highlight that the different responses to light yielded by the optic nerve fibers and caudal photoreceptor can be attributed to differences in physiology, since both nerve fibers were stimulated with the same light intensity
- These results make sense due to the different functions of the each nerve fiber
- The difference in light responses could be attributed to the different mechanisms of the each response
- Unlike the optic nerve fibers, the caudal photoreceptor directly responds to light via their dendrites
- There are no synapses that relay light information to the caudal photoreceptor from the environment (Sullivan and Herberholz, 2013)
- However, the optic nerve fibers rely on prior synapses to receive light information
- Unlike the optic nerve fibers, the caudal photoreceptor directly responds to light via their dendrites
- It is also thought that the difference in response latency between the optic nerve fibers and the caudal photoreceptor is influenced by the different photopigment that is present in each nerve fiber
- The optic nerve fibers use the photopigment opsin
- The photopigment used by the caudal photoreceptor is currently unknown, but as a result of prior studies, it is clear that opsin is not used by the caudal photoreceptor (Kennedy and Bruno; Uttal and Kasprzak, 1962)
Integration of Sensory Modalities
- As a result of the study, the optic nerve fibers were found to have a firing rate more than 10x that of the caudal photoreceptor
- The differences in light response between the two nerve fibers is also encouraged by the different types of information that are processed within close proximity of each nerve fiber
- Optic nerve fibers receive mechanoreceptive information, associated with the eye and head movements, somatosensory input, as well as input from several visual sensations (Wiersma and Yamaguchi, 1966)
- The caudal photoreceptors are just two interneurons that process incoming mechanreception via the ventral nerve cord (Wiersma and Hughes, 1961; Hermann and Olsen, 1967; Wilkens and Larimer, 1976; Kondah and Hisada,
1986; Sullivan and Herberholz, 2013)
- The different firing rates of the optic nerve fibers and the caudal photoreceptor can also be explained by the number of light sensitive cells they are surrounded by
- The increased number of light sensitive cells surrounding the optic nerve fibers, corresponds to increased neural activity, since additional units are firing during light exposure, (Fig. 11)
Challenges of Dissections
- Despite the straight-forward procedure the dissections involve, one must take their time during the dissections and use a stereoscope for precision
- This required patience and precision is particularly relevant when separating the joints between the ocular plate of the protocephalon and a basal sclerite of the eyestalk, during the optic nerve fiber dissection
- It also can be applied to the removal of the integument during the caudal photoreceptor dissection
- If the scalpel blades cut too deeply into the crayfish tissues, then the recordings will not be successful
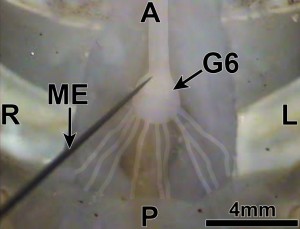
Figure 10: Ventral view of the sixth abdominal ganglion of the ventral nerve cord. Microelectrode (ME) inserted at the location of the cell body of a caudal photoreceptor. A=anterior; =posterior; L=left; R=right. Images here and those shown in Figures 6-9 are from Orconectes immunis, which have integument and connective tissue on the ventral side in the tail that is lighter and somewhat transparent in comparison to Procambarus clarkii, making the ventral nerve cord easier to see during dissection. (Journal of Undergraduate Neuroscience Education)
- When performing the optic nerve fiber dissection, it is advised that the species Procambarus clarkii be used because the joint between the ocular plate of the protocephalon and the basal sclerite of the eyestalk, (where the incision is made), is more apparent in Procambarus clarkii than in Orconectus immunis
- However, when performing the caudal photoreceptor dissection, the species Orconectas immunis proves to yield an easier dissection because the ventral nerve cord is easier to see in Orconectas immunis compared to Procambarus clarkia
- This difference in visibility can be attributed to the lower degree of pigmentation of the integument of Orconectas immunis when compared to that of Procambarus clarkii
Further Experiments
- Once students have learned the dissection and recording procedures, along with the functional topography of the crayfish, they can develop further investigations, such as correlating spectral sensitivities of responses to photopigment absorption spectra or examining how other stimuli, in addition to light, are integrated by optic nerve fibers and the caudal photoreceptor