Variables Measured
- Oxygen concentration
- Measured in g/mL
- Performed with YSI 550A dissolved oxygen meter

YSI 550A Dissolved Oxygen Meter (https://www.ysi.com/550A)
- Water Temperature
- Measure in degrees Celsius
- Salinity
- Measured in parts per million (ppm)
- Conductivity
- Measured in micro Siemens per centimeter (μS)
- Total dissolved solids
- Measured in parts per million (ppm)
- pH
- Turbidity
- Nitrogen concentration
- Measured in grams per milliliter (g/mL)

Oakton PTT estr 35 Multiparameter Meter (http://www.coleparmer.com/Product/Oakton_PCSTestr_35_Waterproof_pH_Conductivity_TDS_Salinity_Tester/EW-35425-10)
- Measured in grams per milliliter (g/mL)
- Phosphorous concentration
- Measured in grams per milliliter (g/mL)
- Salinity, conductivity, total dissolved solids, pH, and turbidity were all measured using an Oakton PTT estr 35 multiparameter meter
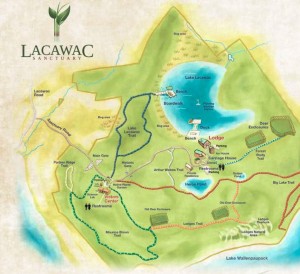
Locations Surveyed
- Pocono Mountains, PA
- Four sites within the Lacawac Sanctuary were surveyed for the study. They include:
- Lake Lacawac-preserved lake
- Acid bog behind Lake Lacawac- Preserved
- Heron Pond (unpreserved)-manmade
- Lake Wallenpaupack (unpreserved public lake)
- Lacawac Sanctuary is a biological sanctuary for ecological research thatincludes several lakes, creeks, and ponds. Lake Lacawac is a preserved lake that is in pristine condition
- Four sites within the Lacawac Sanctuary were surveyed for the study. They include:
- Lehigh Valley, PA
- Hope Road-Swamp/canal
- Sand Island-creek
- Bushkill Creek-creek
- Michigan
- Eight locations including various creeks, lakes and ponds
Survey Methods
- All animals captured were handled according to the Institutional Animal Care and Use Committee (IACUC) at Lafayette College
- Turtles seen in each surveyed location were counted as the experimenter walked along a specific path or observed a specific body of water
- Each species was counted separately
- Turtle species were classified based on the their shell pattern, carapace length, and head coloration
- A high zoom camera was used to view turtles from a distance
- Turtles were captured using hoop nets, stakes, and canned bait
- Hoop nets were placed near the shoreline and opened facing the shore. ¾ of the nets were submerged in order provide the turtles with enough room to breathe
- The nets were placed in locations where turtles appeared to pass through and/or position themselves frequently
- Pictures were taken of each captured turtle’s carapace, plastron, and head
- The time that the net was left in the water was also recorded
- Samples of water were taken using Nalgene containers and brought back to
the lab to obtain measurements for turbidity, nitrogen, and phosphorous concentrations
Retina Preparation for Calcium Imaging
- All animal care and testing obeyed the rules of the Institutional Animal Care and Use Committee (IACUC) at Lafayette College
- Turtles were purchased from Kons Scientific Co., Inc.
- Turtles were kept in 60 gallon tanks with island bricks for sunning
- Room temperature was kept at 23.1°C-36.1°C
- Maximum humidity of room was 88%
- The room was kept on at 12:12 light to dark hour cycle
- Turtles were fed a floating fish food diet every other day and their tanks were cleaned weekly
- 6 Red-eared slider turtles, weighing 0.5-2.5kg were euthanized by injection of pentobarbital into the peritoneum
- By performing the peritoneal injection using pentobarbital, all activity produced by the organs of the turtle was inhibited
- Before euthanasia, the retinas of the turtles were dark adapted for 30 min.
- Exposing the retinas of the turtle to darkness before the dissection, makes it easier to isolate the retinas
- When exposed to light, the cones, within the retina move into the retinal pigment epithelium. This migration of the cones makes it
more difficult to isolate the retina. Therefore, by depriving the retina of light exposure, the cones do not migrate, thus yielding an easier and more successful isolation of the retina
- When exposed to light, the cones, within the retina move into the retinal pigment epithelium. This migration of the cones makes it
- Exposing the retinas of the turtle to darkness before the dissection, makes it easier to isolate the retinas
- After the turtles were euthanized, they were decapitated and each retina was
dissected
- Since there were 6 turtles, there was a total of 12 retinal samples
- Calcium imaging was then performed on each retina
- Each retina was exposed to solutions of different O2 concentrations. The solutions were categorized as anoxic, hypoxic, or normoxic
- The order of exposure to each condition was alternated between
ascending concentration of O2, (anoxic , hypoxic, normoxic), and descending concentration of O2 (normoxic, hypoxic, anoxic), in order to avoid confounding effects
- The order of exposure to each condition was alternated between
- Each retina was exposed to solutions of different O2 concentrations. The solutions were categorized as anoxic, hypoxic, or normoxic
Confocal Imaging
- Each retina was imaged while submerged in each type of solution, using a Nikon E800 C2 microscope
- Confocal imaging was performed with a blue 488nm wavelength laser, close to
the peak wavelength of excitation
- Calcium has been found to be excited by blue 488nm light and it fluoresces at 516nm (Contin et al., 2010)
- Images were captured every 2 sec for a total of 150 counts or 5 min.
- A white light was shown on the retina during the 50th and 100th counts in order to cause photoactivation of the retinal cells
- Under normal or normoxic O2 conditions, it was expected that 15 retinal ganglion cells would be excited, and consequently cause calcium activation
- This calcium activation can been seen with a microscope
- In low oxygen conditions, the retina cells are not expected to perform or activate as well as they normally would, and therefore, yield a weaker calcium activation
- Under normal or normoxic O2 conditions, it was expected that 15 retinal ganglion cells would be excited, and consequently cause calcium activation
- In order to provide a control group to use when averaging the changes in brightness, (changes in calcium activation), for a given cell, a baseline movie was created
- The baseline movie did not include any white light stimulation
- During confocal imaging, 5 retinal ganglion cells per retina, per O2 solution were analyzed