TECHNICAL CONTEXT
Introduction
This section will discuss the technical details of each proposed alternative; biomass gasification, Ensyn’s Renewable Fuel Oil, and the baseline natural gas and fuel oil No. 2 combination. The analysis will include an explanation of the process of using the alternative to produce heat for the college, the characteristics of the fuel itself, materials and equipment necessary for plant operations, associated greenhouse gas emissions, and other environmental concerns. A technical analysis is imperative for a comprehensive economic analysis because many of the values used to create the total cost of each alternative are derived from the technical analysis.
Biomass Gasification
Biomass Gasification involves burning fuel derived from plants such as trees, grass, soybeans, and corn (known as biomass) under restricted air supply for the generation of producer gas (Vermont Family Forests Biomass Assessment Team, 2004). Though there are varying types of gasifiers that are specific to the scale of operations and fuel sizes, the general process involves a key chain of events, shown in Figure 3, that ultimately results in a steam that would be distributed throughout the campus for energy purposes such as heating and cooling.
Figure 3- Biomass Gasification Process Flow Diagram (Source- Themis Power Corporation, n.d.)
Biomass Gasification: The Process
The general process of biomass gasification involves four crucial “steps,” most effectively illustrated in Figure 2 (Basu, 2010, p. 120). The four steps are:
- Preheating and drying
- Pyrolysis
- Char gasification
- Combustion
The preheating and drying process is critical because every kilogram of moisture in the wood chips requires at least 2260 kJ of energy from the gasification process to vaporize the water emitted from the plant, taking away from the energy converted into steam to be distributed out for heating and cooling (Lukac et. al., 2014). High moisture content compromises the wood gas quality and amount of fuel input required. The approximated moisture content of general cut wood ranges from 30-60%s, however for the purpose of gasification, most systems use wood chips with a content of 10-20% for maximum energy efficiency. After the wood chips are pre-heated to reach the optimal moisture content, they are are exposed to extreme heat upon entering the gasifier which releases water. At this point in the process, temperature reaches up to 200 degrees Celsius (Basu, 2010 p. 120).
In the pyrolysis process, wood chips are superheated in a low oxygen chamber, where they smolder and emit wood gas. This process is important because it breaks down large hydrocarbon molecules in the wood chips into smaller gas molecules to prepare them for gasification. After the gasification process is complete and the wood is converted into a synthetic gas (syngas), oxygen is introduced which causes the gas to ignite, producing heat at a temperature of over 600 degrees Celsius to make steam. Once combusted, the resulting flue gas is directed through a boiler and would then be distributed throughout the campus to provide heating and cooling (Basu, 2010, p. 122).
Figure 4- Potential Paths for Gasification (Basu, 2010 p. 119)
The combustion of wood for heat will emit gases and particles into the air, as shown in Figure 4. A critical component of this process is that the exhaust circulates through a cyclone separator and a series of filters which forces larger particles to drop out. The filtration system is estimated to remove 99.5% of particulates, mostly emitting water vapor from the plant. Figure 5 compares the quantity of air pollutant emissions from a coal-based plant to that of a gasification plant and natural gas plant. Major pollutants are particulate matter (PM), Nitrogen Oxide (NOX), and Sulfur Oxide (SOX). While visiting a local biomass plant at a medical center in PA, Lafayette steam plant supervisor Tom Pursel recalls to us his disbelief the plant was running due to the fact that there was barely anything visible rising from the plant.
Benefits of Biomass as Renewable Energy
Biomass is an attractive source of energy because of its ability to reproduce because it is formed from plants and animals. Biomass grows each year through absorbing carbon dioxide (photosynthesis). Thus, when it is burned for energy, it releases carbon dioxide that plants have already absorbed. Through this continuous life cycle, burning biomass does not increase the amount of carbon dioxide emitted into the air since it is consistently being absorbed. Roughly 5% of all biomass, or 13.5 billion metric tons, can be mobilized to produce energy. That amount of fuel is capable of sourcing 26% of the world’s energy consumption and is calculated to be equivalent to 6 billion tons of oil. The use of biomass for energy in developed countries such as the United States is becoming more popular because it is considered carbon-neutral (Basu, 2010).
Furthermore, the benefits of implementing a biomass plant at Lafayette are based on the results seen at Middlebury College, an institution of roughly the same student population and geographical size. Since the construction of their plant in 2009, Middlebury records saving roughly 1,000,000 gallons of #6 fuel oil each year. This is equivalent to 12,500 metric tons of carbon dioxide saved annually and a 40% reduction Middlebury’s emissions as of 2016 (Biomass at Middlebury).
Material and Equipment
The gasification process requires various pieces of equipment that the College would have to acquire. Intuitively, a gasification system is necessary. There are several different types of gasifiers depending on the operating conditions and total volume needed to burn the solid matter (Basu, 2010 p. 168). Gasifiers are generally divided up into two types: ones that have dense phase reactors, the most common types of gasifiers, and lean phase reactors. In dense phase reactors, the wood chips would fill most of the space in the reactor, and areas are designated for specific phases of the process. Several types of dense phase gasifiers are downdraft or co-current, updraft and cross-draft gasifiers, as shown in Figure 6 (Basu, 2010 p. 171-176). Lean phase reactors do not have divisions for the reactions involved in the process, namely the drying, combustion, pyrolysis and reduction; everything occurs in one reactor. The team consultants, plant mechanics and supervisors would most likely provide input on which type of gasifier would be ideal for Lafayette’s intended use.
Greenhouse Gas (GHG) Emissions Calculations
Along with understanding the technical biomass gasification problem, we also calculate the yearly carbon emissions based on an average yearly steam plant energy demand of 122,077.58 MMBtu retrieved from historical data provided by the Lafayette College Office of Sustainability. To ensure comparability between all alternatives, the following conversion factors were used:
Conversion Factors:
- 1GJ= .947817 MMBtu
- 1MWh= 3.412 MMBtu
- 1kg= .00110231 tons
Calculation of social cost of carbon for wood chips:
- Energy content of wood chips: 11 GJ/ton of wood chips (Ciolkosz, D., et al.,2016)
- Social carbon emissions: 6kg/MWh wood chips ((Bates, J. & Henry, S., AEA Group, 2009)
- CO2 emissions: .0226 ton CO2eq/MMBtu
- Lafayette’s annual energy demand: 122,077.5 MMBtu
- 122,077.5 MMBtu*.0226 tons CO2 eq/MMBtu= 2758.9 tons CO2 eq.
As shown in Table we estimate the carbon emissions associated with the wood chip gasification process to be 6kg/MWh wood chips. This data is specific to Canadian forest residues due to lack of accessibility to research performed in the United States (Bates, J. & Henry, S., 2009). Using the conversion factors provided above, roughly .0226 metric tons of CO2 is emitted per MMBtu of wood chips. Lafayette’s energy needs of 122,077.58 MMBtu., therefore producing 2,759 annual tons of CO2 equivalent. Lafayette’s current steam plant using natural gas as its fuel source emits 7,675 annual tons of CO2 equivalent. By implementing a biomass plant on campus, Lafayette will see a 60% decrease in annual emissions.
Table 1. GHG Emissions from wood chip gasification (Source- Bates, J. & Henry, S., 2009)
Renewable Fuel Oil (RFO) / Biocrude
Renewable Fuel Oil, RFO, is a cellulosic biofuel produced exclusively by Ensyn Fuels, a company based in Ottawa, Canada. As a renewable biomass, Ensyn’s RFO feedstock requirements include the following; slash and pre-commercial thinnings from non-federal forest lands, planted trees and tree residue from actively managed tree plantations on non-federal lands, biomass obtained from the immediate vicinity of buildings, public infrastructure and areas regularly occupied by people that are at risk from wildfire, and other activities, including planted crops and crop residue from non-forested agricultural land that is either actively managed or fallow (Gosselin, 2018). Ensyn’s RFO contains predominantly the first two feedstock materials.
RFO Production: RTP Process
Figure 7- RTP Process Diagram (Source- Ensyn Technology Overview, 2015)
Ensyn’s RFO is produced using a unique patented process, RTP, as diagrammed above in Figure 7. RTP is very similar to the Fluid Catalytic Cracking, FCC, process frequently used in refineries converting petroleum to gasoline or diesel. In FCC systems, a catalyst is circulated through a closed loop between a conversion unit and a catalyst regenerator. Ensyn’s RTP system follows a similar methodology, instead circulating inert sand heat carrier instead of the catalyst. More specifically, in the RTP process, the biomass material contacts rapidly flowing hot sand, converting the biomass solid material into vapors, gases and char. Then, the vapors are quenched and recovered as free-flowing light biocrude. The gases and char move to a second vessel where they are reheated and recirculated back to the conversion vessel (Ensyn Technology Overview, 2015). Any remaining gas or char co-products are used as a source of energy to run the Ensyn plant and dry biomass feedstock. Thus, there is no excess energy required for the conversion process, although some electricity is used by the plant for functions such as conveyor belts (Gosselin, 2018).
Technical Considerations of Steam Plant Operations Using RFO
According to Ensyn representative Gregg Gosselin, there are multiple technical implications of operating a steam plant with Renewable Fuel Oil. Additionally, there are technical implications for switching from the current natural gas and fuel oil No. 2 combination to the Renewable Fuel Oil. To begin, the main technical challenge of using RFO is the lower pH value. With a pH of 3, long-term exposure of Renewable Fuel Oil to certain metals can lead to corrosion. For this reason, the surfaces touching the RFO must be stainless steel. This includes the storage tank, any piping, and the boiler itself. Thus, the low pH would require Lafayette to replace its current infrastructure with stainless steel materials (Gosselin, 2018). The costs associated with this project will be discussed in the economic section. However, it is worth noting that Bates College, an institution very similar to Lafayette, has had success in this infrastructure change.
Another technical concern associated with RFO at Lafayette is the water content of the fuel itself. Because Ensyn’s RFO is 20-22% water, the fuel is heavier than comparable fuels, so more frequent deliveries will be required. Currently, only 6,500 gallons of RFO can be delivered per load. With current production facilities located in Canada, this challenge would increase the cost of the fuel. However, Ensyn has taken several steps to mitigate this risk. According to Gosselin, Ensyn would utilize their fleet of railcars to deliver 100,000-120,000 gallons of fuel from Canada to a regional transload facility, where it would be stored until Lafayette’s reserves were depleted (Gosselin, 2018). This strategy will decrease the greenhouse gas emissions associated with the frequent transportation of the RFO from Canada to Easton, PA and increase the long-term reliability of the fuel source.
An additional technical concern is the nitrogen dioxide, NOx, emissions created with the combustion of RFO. The combustion of Renewable Fuel Oil has an emissions factor of .175 lb/MMBtu (HHV) of NOx, enough to require a change in emissions permitting. However, Greg Gosselin noted that emissions permitting has not be a challenge for any other Ensyn projects. Beyond emissions permitting, NOx is concerning because of its global warming implications. NOx, specifically NO2 “is not only an important air pollutant by itself, but also reacts in the atmosphere to form ozone and acid rain” (Clean Air Technology Center, 1999). Additionally,
“Tropospheric ozone has been and continues to be a significant air pollution problem in the United States and is the primary constituent of smog. Large portions of the country do not meet the ozone NAAQS and thereby expose large segments of the population to unhealthy levels of ozone in the air. NO2 reacts in the presence of air and ultraviolet light (UV) in sunlight to form ozone and nitric oxide (NO). The NO then reacts with free radicals in the atmosphere, which are also created by the UV acting on volatile organic compounds (VOC). The free radicals then recycle NO to NO2. In this way, each molecule of NO can produce ozone multiple times” (Clean Air Technology Center, 1999).
Thus, while in the context of the Climate Action Plan, the emission of NOx by Renewable Fuel Oil does impact the college’s carbon neutrality goals, this greenhouse gas does play an important and concerning role in global warming.
GHG Emission Calculations
The final technical concern associated with Ensyn’s Renewable Fuel Oil is the carbon dioxide emissions. In calculating these emissions, we used data supplied by Ensyn’s Northeast Regional Sales Manager, Greg Gosselin. When only using the fuel combustion emissions, we found that a switch from the current combination of natural gas and fuel oil No. 2 would yield a 99.5% reduction in emissions. With such a drastic reduction, we were very skeptical of this figure. Thus, we have created a range of possible GHG emission reduction levels; the aggressive reduction estimate utilizes the GHG emissions from combustion only, as detailed in the figure below, but a conservative estimate results utilizing a more comprehensive life cycle analysis provides a lower bound for our estimate.
According to a Comparative Analysis using GHGenius Software, Ensyn reports that from fuel combustion alone, PyOil (i.e. RFO) produces 301 grams of CO2 equivalent per GigaJoule of energy produced, as seen in the figure below (Gosselin, 2018). It is important to note that this statistic is not the emissions from a lifecycle analysis, which would include emissions data from all aspects of RFO production and use, some of which is outside of the scope of the college’s carbon accounting practices. However, This is significantly lower than combustion carbon dioxide emissions Natural Gas’ (60,762 g CO2 eq/GJ) as well as the combustion emissions from Crude Oil (89,508 gCo2 eq/GJ).
The Comparative Analysis completed by (S&T)^2 Consultants Inc. and referenced by Greg Gosselin does include more comprehensive lifecycle analysis data. When greenhouse gas emissions from fuel dispensing, distribution & storage, production, transmission, recover, land-use changes and cultivation, fertilizer manufacturing, fas leaks & flares, CO2 and H2S removed from natural gas, displaced emissions, and fuel combustion is accounted for, Table 2 displays a grand total of 11,091 g CO2 equivalent/ GJ.
Table 2. Lifecycle GHG Emissions (Source- Gosselin, 2018)
To calculate the emissions from RFO use at Lafayette, an average yearly steam plant energy demand of 122,077.58 mmBtu was assumed, based on historical data. To make the conversion between mmBtu and g CO2 equivalent/GigaJoule, and then eventually to Metric Tons of CO2 equivalent (done in order to compare carbon emissions across all alternatives considered), the following conversion factors were used:
- 1 metric tonne= 1,000,000 grams
- 1 MMBtu= 1.05587 GigaJoules
Based on our aggressive estimate of GHG emission reduction, we found that the combustion of RFO for heating purposes yields about 0.00031782 metric tons of CO2 equivalent per MMBtu. Based on Lafayette’s annual heating needs, this would result in 38.77644908 Metric tons of CO2 equivalent emissions from fuel use alone. With our more conservative approach, the lifecycle GHG emissions of RFO is 0.01171065417 metric tons of CO2 equivalent per MMBtu. This results in 1428.788575 MT of CO2 equivalent emissions from the use of RFO at Lafayette. We use the following summarized values in our economic analysis:
- RFO Aggressive GHG Emission Estimate: 38.77644908 MT of CO2 equivalent
- RFO Conservative GHG Emission Estimate: 1428.788575 MT of CO2 equivalent
These reduction in emissions represent a 99.5% (aggressive estimate) to 81% (conservative estimate) reduction in emissions compared to our natural gas/ fuel oil No. 2 baseline.
Natural Gas
Similar to crude oil, natural gas is extracted by drilling. In the 1970s, the resource was considered too expensive to drill for on its own and was treated as a bonus byproduct of crude oil drilling. Natural gas is a result of “layers of sediment and plant and animal matter that slowly built up…until the pressure and heat resulting from the weight of the overlying sediment eventually converted this organic matter into natural gas,” (Curtis, 2008). Natural gas is a tradable commodity, satisfying “24 percent of the nation’s energy demand (consumption), moving ahead of coal,” (Potential Gas, 2008).

Figure 8. Schematic Geology of Natural Gas Resources (Source: U.S. Energy Information Administration)
However, hydraulic fracturing, otherwise known as fracking, has allowed drillers to access a significant amount of natural gas previously deemed nonexistent. Philip Budzik, a research analyst at the Energy Information Administration, says fracking is a large reason for the low price of natural gas, saying “In 2008 it was producing maybe half a billion cubic feet per day, and now it’s producing something like 16 billion cubic feet per day…What happened was trillions of cubic feet of gas that we thought weren’t extractable [now is],” (Investopedia). The process occurs by drilling into shale rock and injecting a mixture of water, sand, and chemicals to create tiny fractures into the ground, that access pockets of oil and natural gas. This technological advancement significantly increased the supply of natural gas, beginning the fracking revolution.
![]()
![]()
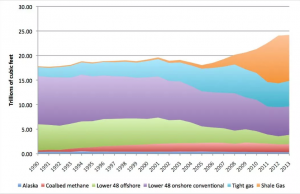
Figure 9. The significant rise of Shale Gas supply as a result of fracking practices (Source: Investopedia)
Due to the large supply, natural gas is considered reliable over the long term, though still requires a second fuel to be used as a backup. In terms of national supply, “technically recoverable natural gas…is more than 1,744 trillion cubic feet (Tcf) (50 km3 ), which includes 211 Tcf of proven reserves,” (Kargbo, et. Al). To put this total in perspective, this figure suggests “there is enough natural gas to supply the U.S. for the next 90 years.” The college’s source of natural gas is processed and supplied by locally by Metropolitan-Edison, an energy company and subsidiary of First Energy, one of the largest in the country. Geographically, Lafayette College is located near the Marcellus shale, one of the largest areas of natural gas extraction. The logistical infrastructure to support natural gas is already in place for the school’s continued use. Natural gas is delivered from Metropolitan Edison through a pipeline system with interruptible service. However, natural gas is not without reliability risk. When local supply of natural gas is not enough to meet demand in the local area, the plant may temporarily shut down the school’s access to natural gas. To hedge this risk, the school uses fuel oil number two as a backup. The fuel is locally stored, with roughly 20 deliveries made by truck each year (DeSalvo, personal communications, 2018).
Table 3. Quantity of fuel used by Lafayette in 2017-2018
(Source: DeSalvo, personal communication, 2018)
| 2017-18 Fiscal year – Gallons of Fuel Oil | Volume of Fuel per Truck (gallons) | Total Annual Deliveries |
| 79,042 | 4000 | 19.8 |
When natural gas is accessed by the heating plant, the fuel is processed in the school’s boilers. Natural gas is heated to create steam, most of which exits through a pipe distribution system to each of the buildings on campus. The remaining steam moves through a series of heat exchangers, which allows the gas to condense. The waste gas exits through a stack, while simultaneously allowing heat to be recirculated, allowing the boiler to be more efficient in heating up the natural gas. The byproducts of natural are water, methane, nitrous oxide, and carbon dioxide.

Figure 10. Diagram of Natural Gas Boiler
Greenhouse gas emissions are measured in metric tonnes of carbon-dioxide equivalent, a metric that considers how much equivalent damage is done to the environment by different chemicals. Calculating this figure requires the amount of annual energy required by the school, the carbon-dioxide equivalent conversion factor, and how much natural gas and fuel oil number two was consumed.
Conversion Factors:
- Natural Gas Emissions Factor: 0.0531145 tonnes CO2 equivalent/MMBtu
- Fuel Oil Number 2 Emissions Factor: .075343 tonnes CO2 equivalent/MMBtu
Calculation of tonnes CO2 equivalent/MMBtu
- 107,243.22 MMBtu of Natural Gas * 0.0531145 ≈ 5696 tonnes CO2 equivalent
- 14,834.36 MMBtu of Fuel Oil Number 2 * 0.075343 ≈ 1118 tonnes CO2 equivalent
- Total tonnes CO2 equivalent = 6814 tonnes
Conclusion
The technical analysis section of this report discusses the characteristics of the fuels, the processes used to produce them and utilize them for heating purposes, and any infrastructure changes that would be required at Lafayette. Additionally, calculations of the total greenhouse gas emissions from the respective alternatives will play an important role in our economic analysis and work to address the college’s primary concern of reducing emissions, institutions including Middlebury College and Bates College will soon and have already done.
To view our economic analysis, click here:Economic Context (Biogenics)