CHE 324 Process Controls Project – Kegerator
Kegs have played an integral role in the storage, transportation and dispensing of beverages for many years. Historically, kegs were constructed using wood but nowadays they are typically made of stainless steel. A keg, or half-barrel is a 15.5 U.S. gallon vessel. A quarter-barrel has a volume of 7.75 U.S. gallons. Generally a keg is a vessel smaller than a barrel; thus, it is 30 gallons or smaller. A manual pump is used to generate pressure, pushing the beverage out of a hose. Keeping the liquids somewhat insulated, and allowing easy access; a keg is a great way to serve beverages at social gatherings.

Figure 1 – Inner view of a Keg
Unfortunately, kegs do have a few shortcomings. While they do provide some degree of insulation, they cannot cool the beverage or control the temperature. A traditional keg also requires manual pumping, which can be tiresome and waste time. These issues can be solved with a Kegerator, a specialized refrigerator built to work with kegs. Kegerators are generally designed for use with kegs, but they are gaining popularity for dispensing other types of drinks, most notably wine, cold brewed coffee, kombucha, and soda. With home brew kegs, you can put whatever liquid you want inside the keg, pressurize it and dispense it with a kegerator. Different types of liquids require different alterations to the dispense system. Wine and cold brewed coffee use a CO2/Nitrogen blend to pressurize the kegs. These types of drinks also require all stainless steel contact with the dispense system fittings because the higher acid content can corrode the chrome plated brass normally found in dispense systems.A kegerator has a built in control system to regulate temperature, working just like a typical refrigerator. To add pressure, a compressed gas tank is attached instead of manual pumping. A gas mixture, high in CO2, is sent through a regulator to keep the contents of the keg at constant pressure, so the beverage will be dispensed when a valve is opened.
A kegerator solves many of the issues associated with a typical keg, but we believe the system could be improved further. With a kegerator, careful care must be taken to maintain proper levels of dissolved carbon dioxide. Typically, a user first chooses a temperature as a set point for the system. At that given temperature, the regulator pressure must be set to a specific value to ensure the right amount of carbonation in the beverage. This is not the most direct way of controlling CO2 levels, and also means that the user cannot adjust pressure levels as they like to alter the flow rate of the beverage out of the keg. Because of this, at low pressure settings the beverage is pumped out too slowly for a social gathering with many people and at high pressure settings the turbulent flow out of the hose causes too much foam.

Figure 2 – Kegerator
We believe that with a few modifications to the design, and an added control system, we could make improvements to the Kegerator. These improvements would would exponentially enhance the experience of the consumers, as CO2 increases the life of the beverage and temperature further enhances its taste. Further temperature has a formidable effect on both viscosity and solubility of the liquid.
As the temperature of a solution is increased, the average kinetic energy of the molecules that make up the solution also increases. This increase in kinetic energy allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions. The average kinetic energy of the solute molecules also increases with temperature, and it destabilizes the solid state. The increased vibration (kinetic energy) of the solute molecules causes them to be less able to hold together, and thus they dissolve more readily. The impact of increasing temperature will be to slow down the sphere in gases and to accelerate it in liquids. When you consider a liquid at room temperature, the molecules are tightly bound together by attractive inter-molecular forces (e.g. Van der Waal forces). It is these attractive forces that are responsible for the viscosity since it is difficult for individual molecules to move because they are tightly bound to their neighbors. The increase in temperature causes the kinetic or thermal energy to increase and the molecules become more mobile. The attractive binding energy is reduced and therefore the viscosity is reduced. If you continue to heat the liquid the kinetic energy will exceed the binding energy and molecules will escape from the liquid and it can become a vapor.
Typically, a gas will increase in solubility with an increase in pressure. This effect can be mathematically described using an equation called Henry’s Law. When a gas is dissolved in a liquid, pressure has an important effect on the solubility. William Henry, an English chemist, showed that the solubility of a gas increased with increasing pressure. He discovered the following relationship:
C = k * Pgas
In this equation, C is the concentration of the gas in solution, which is a measure of its solubility, k is a proportionality constant that has been experimentally determined, and Pgas is the partial pressure of the gas above the solution. The proportionality constant needs to be experimentally determined because the increase in solubility will depend on which kind of gas is being dissolved.
The goal of this control system would be to keep temperature and dissolved carbon dioxide levels in the beverage (the controlled variables) as close as possible to a desired set point, as well as to give the user the ability to adjust the pressure in the keg as desired. Operating ranges for temperature and CO2 level are based off of the user’s preferences. Guidelines for these are often provided by the manufacturer of the beverage. While controlling this system is very critical for it to function, it will add enjoyment to the user’s experience.
We plan on optimizing the Kegerator by adding a nitrogen tank in addition to the existing carbon dioxide tank. Both tanks will have a valve (that can be turned to varying degrees of openness) linked to a controller. After the valve, both tanks will have a regulator (set to the same pressure). These lines will both feed into the keg. A sensor to detect dissolved carbon dioxide in the beverage can be placed inside the keg. The refrigeration unit acts exactly as a typical mini-fridge. A display on the outside will allow the user to specify a set point for temperature and dissolved CO2 levels. Therefore, this system would employ a feed back loop, wherein the temperature and CO2 levels in the kegerator would be constantly monitored. If these values fall outside the user’s preference, the manipulated variables would be signaled. The major advantage of such a feed back system is that it’s reactive to disturbances in the surroundings, as it automatically compensates for any such differences by manipulating the required variables.
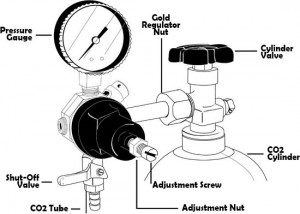
Figure 3 – CO2 Regulator
For this system, manipulated variables are the electrical power sent to the compressor and the valve positions for the nitrogen and carbon dioxide tanks. Pressure in the tank could possibly be considered another controlled variable and in this case, the manipulated variable would be the regulator openness, with the user acting as the controller.
A few disturbance variables exist for this application, too. Changes in temperature surrounding the system could affect the temperature of the contents of the keg. Changes in outside temperature could also affect the pressure and flow rate of the gas from the tanks. Finally, changes in altitude could affect pressure and the desired carbon dioxide levels in the beverage.
References
“How to Make Cold Brew Iced Coffee.” How to Make Cold Brew Iced Coffee. N.p., n.d. Web. 31 Mar. 2016.
“Solid Solubility and Temperature – Boundless Open Textbook.” Boundless. N.p., n.d. Web. 31 Mar. 2016.
“How Does Temperature Change Viscosity in Liquids and Gases?” How Does Temperature Change Viscosity in Liquids and Gases? N.p., n.d. Web. 31 Mar. 2016.
At its core, unaltered concept, the process of the kegerator does make sense. I think the addition of the CO2 regulator and the temperature control make sense, but am not quite sure what the addition of the nitrogen regulator will do. Most beers have only CO2 dissolved in them, so spending the money to hook up a nitrogen tank to every kegerator doesn’t seem to quite make sense. Granted there are beers that do better with nitrogen, but the number of those beers does not seem to justify the cost of putting the regulator in all kegerators. I can definitely see the general public using the upgraded version of the kegerator. As bars already often use the original kegerator, I feel that these upgrades would only improve their customer satisfaction. Not only would the beer be colder, but there would be less of a wait time, and less foam when the beer finally arrives.
The article states the temperature and the dissolved CO2 levels as the controlled variables, which seems to make sense. If they are adding a nitrogen tank and regulator, it would seem that dissolved nitrogen would also have to be a controlled variable. The manipulated variables are stated as the electrical power sent to the compressor and the valve positions for nitrogen and carbon dioxide tanks. Although it makes sense that the electrical power to the compressor is a variable, it does not make sense that the valve positions for nitrogen and CO2 are since nitrogen and CO2 are the controlled variables. I would say that additional manipulated variables are the heat added or taken from the refrigerator. The disturbance variables here, although they make sense, should be fixed by the self-regulation of the new kegerator that monitors and changes the temperature and pressure of the beer.
Although not proposed in the article, a PI controller would be best for the upgraded kegerator. The function of the proportional action in the controller is used to reduce some of the oscillation and instability introduced by the integral action. Additionally, the proportional action will react continuously to error based on what it currently sees in the system (i.e. the current temperature and dissolved CO2 in the beer). In order to reduce offset and push the system more towards the set point, the integral action is added. The integral action is good for fast response to sustained error, therefore, continuously monitoring the temperature and dissolved CO2 is a task for the integral controller. The integral controller will also react to the error based on what is has seen, giving an even more accurate tuning response.
I love the idea for this product. In this day in age, social events are becoming more and more popular among the younger generations. Not only do people hold parties in college, but when they graduate as well. For those who hold big Superbowl parties or large family gatherings, a Kegerator would help keep their beverages cold and fresh. I also think this product is extremely relevant to the college market. Large parties, consisting of multiple kegs, could take advantage of a Kegerator, or multiple Kegerators. I would be interested in purchasing one your products once the new additions of a Nitrogen tank, dissolved gas sensor, and temperature sensor are all added.
I agree with your controls for the system. I think for the temperature control of the Kegerator, the feedback control is the best option. If the Kegerator acts like a mini-fridge, then a sensor can be put on the inside of the fridge to determine the temperature of the surroundings of the keg. The sensor can then be connected to a controller attached to a valve on the cold air inlet line. If the temperature of the fridge falls below the optimal temperature of the beverage in the keg, the valve would close and decrease the cold air flow rate, while if the temperature was too high, the valve would open more.
For the Nitrogen and Oxygen tanks, I like the dissolved oxygen sensor and control system. I am also curious if there is a specific combination of Carbon Dioxide to Nitrogen that provides the freshest of refreshments. If so, the two gas tanks could be connected by a feed forward ratio controller that reads the ratio of gas exiting the two tanks and compares it to a set value.
Since there are different controllers for this process, there will probably be different control schemes. The concentrations of the dissolved carbon dioxide and dissolved nitrogen should be controlled by a PI Controller. The PI controller ensures that the concentrations will eventually reach, or approach, the set point of the controller. If just a P controller was used, there is a lower chance that the concentration of the dissolved gases will reach the correct set point. A PI controller is more specific in reaching the set point. For the temperature of the system, there is most likely an optimal operating range of temperatures for the mini-fridge. The temperature of the keg does not to have to stay at an exact temperature or within a 1-2 degree range. There is room for some variation. That being said, you can probably get away with a Proportional controller on the temperature setting. If you don’t mind a few degree difference from the set point temperature and the actual temperature of your system, a P controller will do the job. If you want to be more accurate and have an actual temperature with little variation from your set point, you could use a PI controller like the dissolved concentration controllers.
I think the improved Kegerator adds a lot of versatility to the original Kegerator. The ability to choose pressures and temperature helps you suit the beverage of choice inside. I think I would not personally buy one, but I think it would be a good investment for bars or places that served tapped drinks. The addition of nitrogen is a good idea to prolong the life of drinks that go bad and can save the company money and time in the long run. Also the control would probably stop drinks from becoming too foamy, which is a real plus. I am not sure how much more expensive this would be, but I can see it being a must have especially if it improves taste and longevity.
I agree with the manipulated variables. Temperature is pretty straight forward as a controlled variable, which was part of the old system. Adding the pressure/solute CO2 as another controlled variable fits the idea of the new system. To make all these changes I would agree that is necessary to control the valves and power of the compressor. It might be important to reiterate the distinction between the CO2 canister and the solute CO2. I think it was forgotten to mention that temperature is still being controlled, so another manipulated variable would be the energy going out of the keg due to the cool air surrounding the keg. As far as disturbances go, I agree with what was said. Changes in outside temperature are important. I think the amount of liquid left inside of the keg could have an impact on how the temperature changes. Power outages and the canisters running out of gas could change how much the process can react. If the keg was shaken prior to being put in the Kegerator, it could affect the solute CO2. I think the beverage would lose carbonation over time which would also be a disturbance.
I agree that a feedback loop would be the best way to control the process. I think that a PI controller would be best. PI is enough to give a fast enough response and to reach the set point. The process itself is not going to have fast changing disturbances, so PI would be just fine. I think tuning would be done on a closed loop basis. This is because the process is pretty boring without control (the drink just gets to room temperature) and I think it would be easier to understand how the Kegerator works with control in action. I think it would also be important to develop controller setting based on what drink is being controlled. This way if it is wine instead of beer it would not be pumped full of unneeded CO2. This adds flexibility to the process. In all I think you guys did a good job explaining the needs and science behind the process.