|
B.S. in Chemistry: | Messiah College | |
| Ph. D. in Inorganic Chemistry: | Iowa State University | ||
| Post Doctoral: | University of Vermont | ||
| Teaching Interests: | General Chemistry
Inorganic Chemistry |
||
| e-mail: | nataroc@lafayette.edu | ||
| Phone: | (610) 330-5216 | ||
| Fax: | (610) 330-5714 | ||
| Office: | 236 Hugel Science Center | ||
| Lab: | 223 Hugel Science Center |
Research Interests
The primary focus of my research is investigating the chemistry of phosphinometallocenes (Figure 1). One of the most widely studied
Figure 1. A generic phosphinometallocene
compounds in this family is 1,1′-bis(diphenylphosphino)ferrocene, commonly called dppf, in which M = Fe and R = Ph. In addition to dppf there are many closely related ligands in which the metal (M) and/or the R groups can be changed to vary the properties of the ligand. These bidentate phosphines are primarily used as a ligand bound to various transition metal centers. Many of these compounds have applications in catalytic reactions.
Studies in my lab examine the fundamental properties of these phosphinometallocene ligands. Of particular interest is the electrochemistry of the free ligands and compounds containing these ligands. The most common electrochemical technique employed in my lab is cyclic voltammetry. Typically this is performed on the benchtop using a CH Instruments potentionstat (Figure 2), but we can also do electrochemistry in the glovebox (Figure 3).
 Figure 2. Electrochemistry equipment Figure 2. Electrochemistry equipment |
 Figure 3. Glovebox Figure 3. Glovebox |
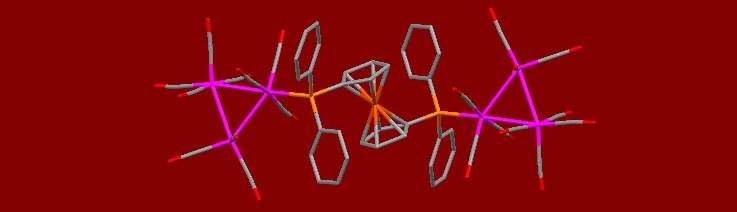
In the course of these studies, numerous transition metal complexes of these ligands as well as a variety of phosphine chalcogenides have been prepared. Typically the synthesis requires inert atmosphere techniques (Figure 4) and stringent solvent purification (Figure 5). The new compounds are characterized by NMR (Figure 6) and in many cases X-ray crystallography. While I’ve been fortunate to work with some great collaborators at Villanova University and The College of New Jersey in the past, we have acquired our own diffractometer (Figure 7) and are now happy to pay it forward and run structures for others. The first crystal structure obtained in the group was by Abby O’Connor and it was the compound [Ru3(CO)11]2[μ-dppf] which is shown in the header for this page.
 Figure 4. Schlenk line Figure 4. Schlenk line |
 Figure 5. Solvent system Figure 5. Solvent system |
 Figure 6. NMR Figure 6. NMR |

Figure 7. X-ray diffractometer |
All of this work has been possible because of the tremendous efforts of the undergraduate students I have had the honor to work with. I firmly believe that a quality undergraduate research experience is a crucial part of my educational mission. Students are co-authors on all of my papers from Lafayette. I do my best to follow the careers of my research students once they have left Lafayette and you can catch up with them using the links above. You can also explore some of the PR Lafayette has chosen to post about my students.
Educational Interests
I am a member of the administrative team for the Interactive Online Network of Inorganic Chemists

and serve as a site administrator for the Virtual Inorganic Pedagogical Electronic Resource.
I am member of the editorial board of the journal Transition Metal Chemistry.
I am also a member of the advisory board of the Covalent Bond Classification website.