FUNDING:
NIH R15 Academic Research Enhancement Award (AREA) Grant (1R15GM148) – (2023-2026)
RESEARCH OVERVIEW:
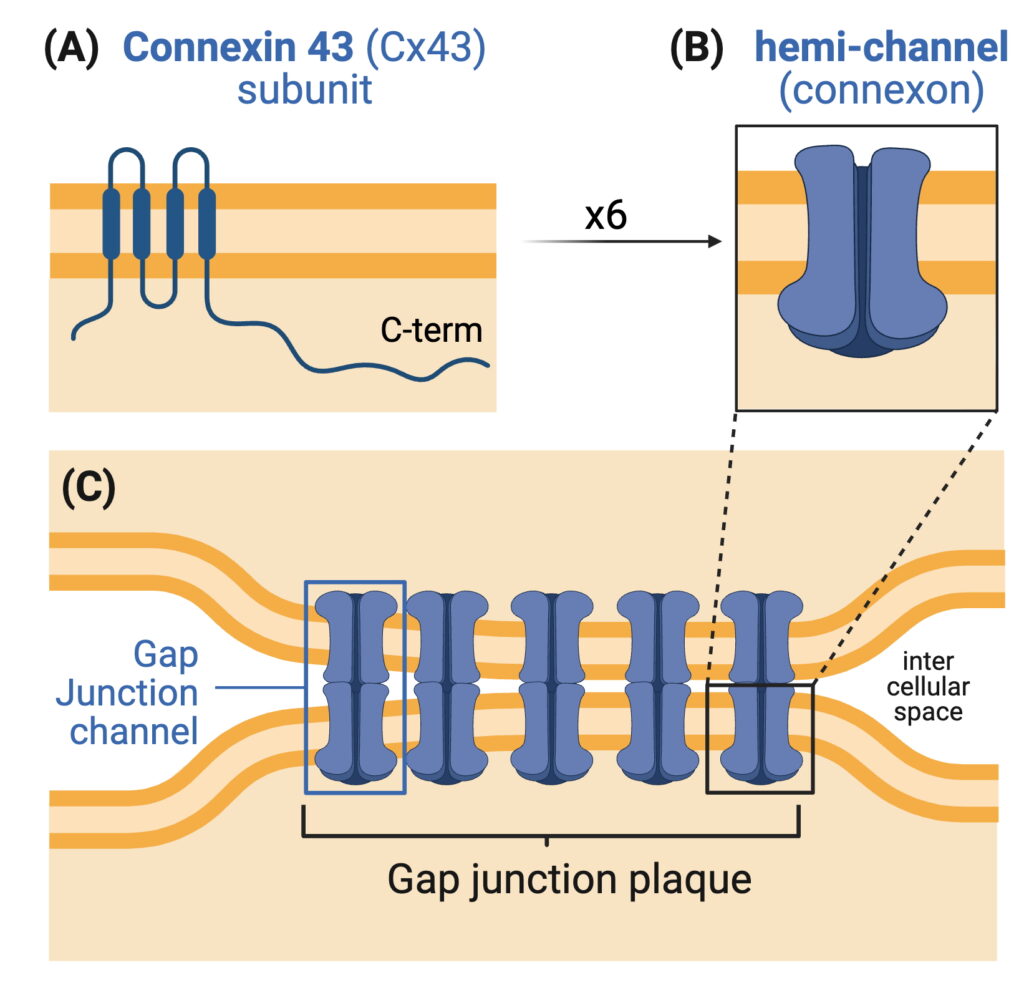
Connexin 43 (Cx43) is a protein constituent of gap junctions (GJs), cellular structures that provide direct cell-cell communication within tissues by allowing small molecules and ions to move between adjacent cells. Mis-regulation of GJs is known to lead to various disease states, such as heart disease, cancer, deafness, and many others. Cx43 function is regulated through phosphorylation (an addition of a phosphoryl group onto serine (S), threonine (T), or tyrosine (Y) residues) by enzymes called protein kinases. There are over 15 known phosphorylation sites on Cx43 modified by several kinase families. Phosphorylation is key to proper Cx43 folding/trafficking through the secretory pathway, GJ formation at the plasma membrane, GJ opening and closing, GJ internalization, and their degradation through endolysosomal and autophagosomal pathways.
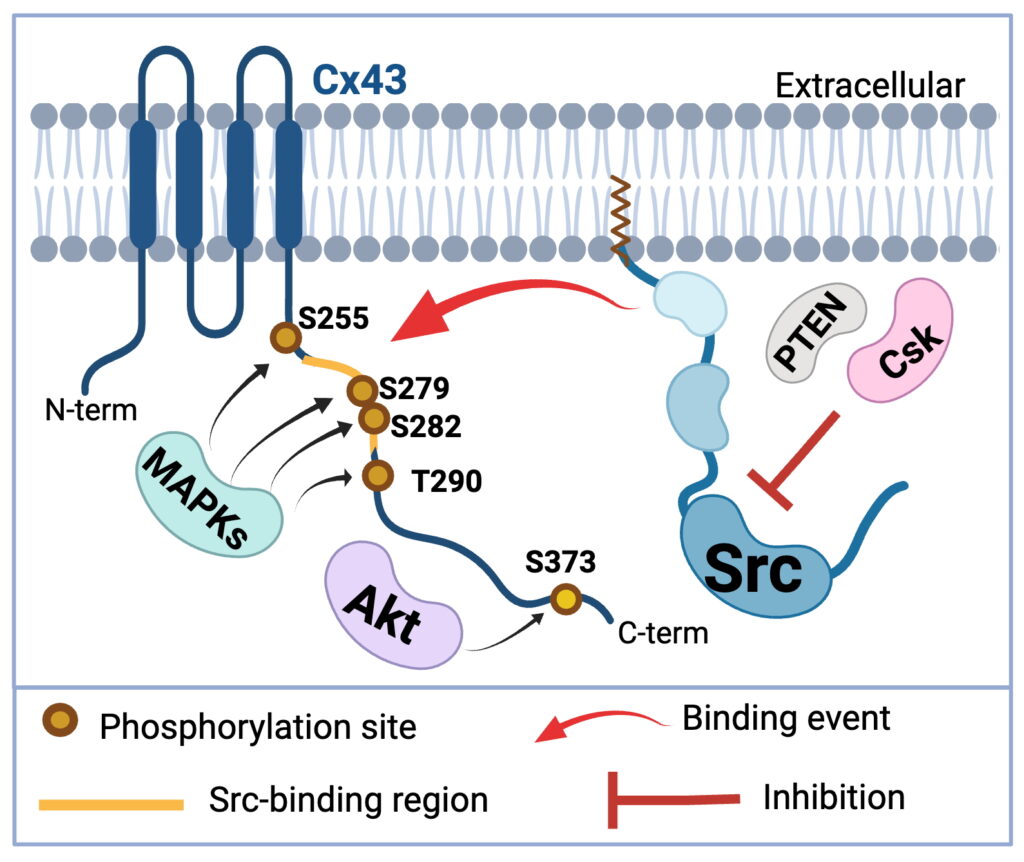
Cx43’s role extends beyond GJ intercellular communication. Cx43 can act as an intracellular signaling hub by interacting with crucial signaling molecules such as Src, a protein that regulates cell proliferation and cancer progression. When Src is recruited to Cx43 at the plasma membrane, two of Src’s inhibitors (enzymes Csk and PTEN) are then able to inhibit Src’s oncogenic activity. We have proposed the regulatory mechanism of Src activity driven by Cx43 phosphorylation on several sties: S255, S279, S282, T290, S373. We are utilizing this knowledge in developing Cx43-based peptides for use as Src inhibitors in cancer cells.
ONGOING PROJECTS:
Regulation of Gap Junction Function through Phosphorylation by Mitogen Activated Protein Kinases (MAPKs)
Effects of ERK, JNK, and p38 Signaling on Connexin 43-Src Interaction
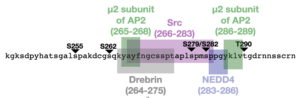
Our recently published work (Latchford et. al., 2025) on Cx43 phosphorylation by MAPKs identified that each member of the MAPK family (ERK, JNK, and p38) has a differential specificity toward Cx43. We used purified and cellular versions of ERK, JNK, and p38 and assessed differences in Cx43 phosphorylation. In addition, we discovered a novel ERK phosphorylation site, (T290). We are currently studying the effects of each MAPK on Cx43-Src interaction, as well as the ability of these phosphorylation events to regulate interactions with cytoskeletal components (through Drebrin), and internalization machinery (Nedd4 and AP2) in the context of GJ function.
Effects of Cx43 Phosphorylation by Akt on on Cx43-Src vs. Cx43-ZO-1 Interactions
It has been demonstrated previously that GJ formation at the plasma membrane is regulated by a scaffolding protein, Zona Occludens 1 (ZO-1). Intriguingly, ZO-1 and Src are unable to interact with Cx43 GJs simultaneously. We are currently assessing Cx43 phosphorylation/dephosphorylation events that regulate these mutually-exclusive interactions.
Development of Cx43-based Peptides to Target Src-driven Cancers
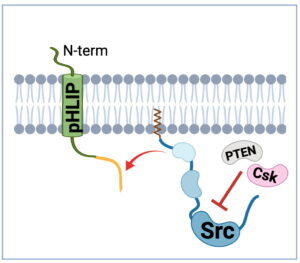
Our work in testing the hypothesis that Cx43 phosphorylation on multiple sites functions as a regulatory mechanism for the interaction with Src, is informing the design of Cx43-based peptides that could be used in targeting Src in cancer cells. To selectively deliver and anchor Cx43 sequences at the cytoplasmic leaflet of the plasma membrane where active Src is localized, we are utilizing pHLIP (pH Low Insertion Peptide).