Important NMR Terms
Baseline: the straight line that appears at zero intensity where there is nothing to observe (no peak).
NMR: nuclear magnetic resonance
Spectrum: (plural, spectra) series of absorbances. For NMR spectrum refers to the plot of radio frequencies absorbed by your sample. Typically represented as a graph of relative absorbance (x-axis) versus chemical shift in ppm (y-axis).
Chemical shift (δ): position of a nucleus signal in an NMR spectrum resulting from its molecular environment. Chemical shift is measured in parts per million (ppm) as a function of its distance away from a standard (typically TMS).
\(\text{Chemical shift} = \frac {\text{position of signal} – \text{position of TMS}} {\text{spectrometer frequency}} \cdot 10^6 = ppm\)Downfield: (or deshielded) left of relative position on an NMR spectrum.
Upfield: (or shielded) right of position on an NMR spectrum.
Resonance frequency: radio frequency at which a nucleus is induced to spin-flip. Used commonly as the peak position in an NMR spectrum.
Multiplicity: (or spin-spin splitting) number of peak into which a signal is split. In 1H NMR a signal will be split n+1 times, where n is equal to the number of neighboring protons present in the molecule.
Integral: the area taken up by a signal. The integrated area is proportional to the number of nuclei represented by that signal. Integrals represent the relative concentration of each nuclei within the sample; absolute concentration can only be determined if a standard of known concentration is introduced into the sample.
| Chemical Equivalence: two atoms are chemically equivalent if, and only if, their environments are identical. Chemically equivalent nuclei will have the same resonance frequency.
Magnetically Equivalent: two nuclei are magnetically equivalent if, and only if, they couple to all other nuclei in an identical manner. |
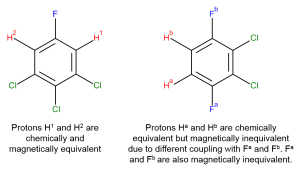 |
Standard: substance to which all chemical shifts are compared. TMS (tetramethylsilane) is the most common internal standard.
TMS: – tetramethylsilane is the typical internal standard used in NMR. The 12 protons are all chemically equivalent and resonate at a frequency lower than most protons in organic compounds. This is the reason the TMS signal is referenced to a value of zero ppm. Most organic protons are less shielded and will appear downfield of zero; however, NMR peaks with negative values are not considered unusual.
Spin Flip: change in the orientation of the nuclear magnetic moment in relationship to the external magnetic field. This is caused by either absorption of energy (excitation) or emission of energy (relaxation).
Splitting: due to spin-spin coupling NMR signals are often separated into groups (multiplets) of two or more. Common splitting groups are doublets (d), triplets (t), quadruplets (q), sextets, septets, and multiplets (m). If spin-spin coupling exists with two or more non-equivalent sets of protons the resonance will be split by each group independently and will appear as a complicated splitting pattern such as a doublet of doublets (dd), triplet of doublets (td), etc.
Spin-Spin coupling: interaction between spin magnetic moments of nuclei (in NMR). Spin-spin coupling gives rise to splitting (or multiplets) observed in many NMR spectra.
Coupling constant (J): spacing between lines in a splitting pattern, typically given in Hz.
FID (Free Induction Decay): the radio frequencies detected in a pulse NMR experiment as excited nuclear spins relax to their lower energy states.
FT (Fourier Transform): a mathematical transformation used to convert the FID signal from the time to the frequency domain.
Radio Frequency: (Rf) the range of electromagnetic radiation with a smaller frequency than infrared, ranging from 3 kHz to 300 GHz. Spin flips in the magnetic fields induced by NMR spectrometers occur in this energy range.