NMR spectroscopy can be used to measure the magnetic properties of a variety of different nuclei. Within the context of organic chemistry it is typically used to determine the number, type, and relative location of 1H (hydrogen atoms) and 13C (carbon atoms) in a molecule. This utilization will be the main focus of this site. However, NMR is a very powerful tool and is used to investigate a large variety of different nuclei within the chemical, biochemical, and medical fields. Any nuclei with an odd number of protons, an odd number of neutrons, or both has quantized spin angular momentum and can be detected using NMR. Nuclei meeting these conditions have a non-zero nuclear spin quantum number (l) and can therefore be detected by an NMR spectrometer. Only nuclei with l=0 are invisible by NMR.
The nuclear spin quantum number (l) for both 1H and 13C nuclei is ½. 12C nuclei cannot be studied using NMR because it has a nuclear spin quantum number of zero. The table below displays common nuclei isotopes and their nuclear spin.
| Natural % Abundance | Nuclear Spin | |
| 1H | 99.985 | 1/2 |
| 2H | 0.015 | 1 |
| 9Be | 100 | 3/2 |
| 10B | 19.6 | 3 |
| 11B | 80.4 | 3/2 |
| 13C | 1.11 | 1/2 |
| 14N | 99.6 | 1 |
| 15N | 0.37 | 1/2 |
| 17O | 0.037 | 5/2 |
| 19F | 100 | 1/2 |
| 31P | 100 | 1/2 |
| 103Rh | 100 | 1/2 |
Ebsworth, E.A.V.; Rankin, D.W.H; Cradock, S. Structural Methods in Inorganic Chemistry Second Edition; CRC Press: Boca Raton, Fl 1991 pg 31-33.
What is the origin of NMR signals?
We will restrict our conversation to nuclei with spin quantum numbers of ½ in this section.
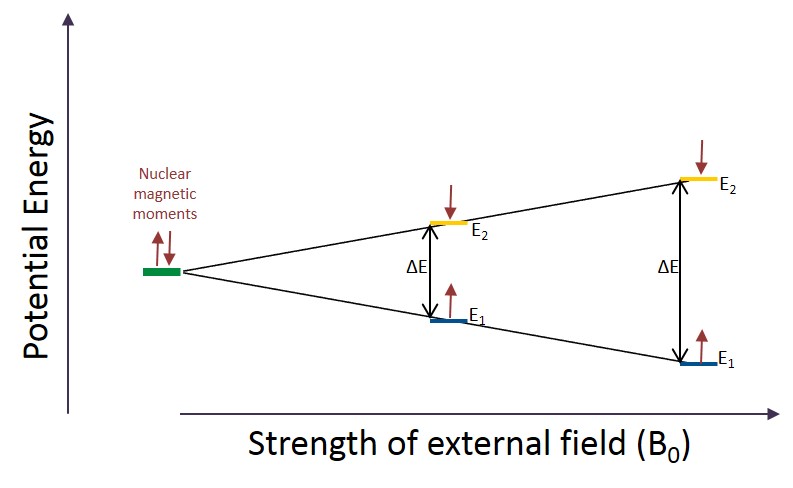
The two allowed spin orientations for a nuclei with l=½ is clockwise (l=+½) and counterclockwise (l=-½). In the absence of an external magnetic field, these orientations are energetically degenerate and therefore equally occupied. This phenomenon is displayed in figure A. Notice that before the external magnetic field has been applied both of the nuclear magnetic moments have the same relative potential energy. However, once an external magnetic field is applied to a sample the degeneracy of the two spin states is broken. Nuclei with spins aligned (up, l=+½) with the external magnetic field will be lower in energy than the nuclei with anti-parallel spin (down, l=-½). The size of the energy gap between the low energy state (E1) and the high energy state (E2) is relative the strength of the applied magnetic field (B0). The gap in energy is relatively small; however, it does cause the lower energy state (E1) to be slightly more populated than the high energy state (E2).
Note: The magnetic moment of each proton is responsible for the break in degeneracy. Any spinning charge creates a magnetic moment. The magnetic moments of the nuclei with l=+½ will be aligned with the external magnetic field and the moments of the nuclei with l=-½ will be opposed to the external magnetic moment creating a small energy gap between the two states.
Nuclei that occupy the lower energy state (E1) can absorb a quantum of energy to be excited to the higher energy state (E2). The electromagnetic radiation required to cause this spin transition (also called a spin-flip) falls within radio frequencies. When a spin-flip occurs the nuclei are said to be in resonance with the applied radiation (radio frequency). This is the origin of the name Nuclear Magnetic Resonance (NMR) spectroscopy.
The relaxation of the nuclei back to E1 releases quanta of energy that can be detected by the instrument as a free-induction decay (FID) signal. This FID signal is computationally transformed into the spectrum of resonant frequencies using a mathematical method called Fourier Transform (FT).