MULTIPLICITY
High resolution NMR spectrometers can reveal a phenomenon called spin-spin splitting (or 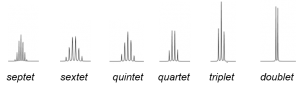 spin-spin coupling) between non-equivalent hydrogen atoms on neighboring carbon atoms. The multiplicity or splitting of each peak can be explained empirically using the n+1 rule. Each group of equivalent protons can sense the number of protons (n) on a neighboring carbon atom and its peak is separated into n+1 peaks.
spin-spin coupling) between non-equivalent hydrogen atoms on neighboring carbon atoms. The multiplicity or splitting of each peak can be explained empirically using the n+1 rule. Each group of equivalent protons can sense the number of protons (n) on a neighboring carbon atom and its peak is separated into n+1 peaks.
| # of equivalent protons to which nucleus is coupled | Name of multiplet | Line intensity |
| 1 | Doublet | 1:1 |
| 2 | Triplet | 1:2:1 |
| 3 | Quartet | 1:3:3:1 |
| 4 | Quintet | 1:4:6:4:1 |
| 5 | Sextet | 1:5:10:10:5:1 |
| 6 | Septet | 1:6:15:20:15:6:1 |
Notice the line intensities of multiplets correlate to the coefficients of a binomial expansion. Go to the Multiplicity Theory page for an explaination of why the line intensities correspond to Pascal’s triangle.
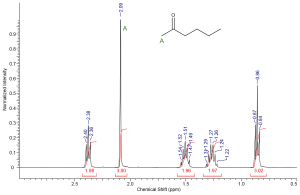
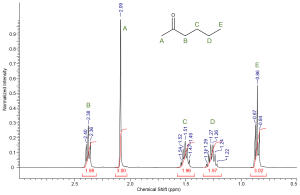
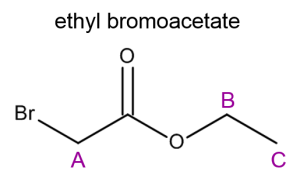
Using the spin-spin coupling rule (n + 1) we can predict the multiplicity of each resonance based on the structure of a molecule. Try to determine the splitting patterns for each set of protons in ethyl bromoacetate. Then test yourself; can you sketch a rough NMR spectra for ethyl bromoacetate? Make sure to include chemical shift, integration and splitting into your answer. Label each peak appropriately.