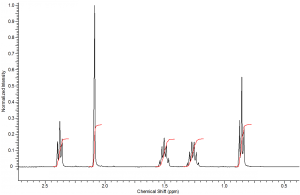
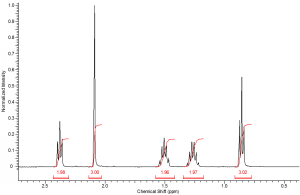
Each peak of an NMR spectrum will be integrated by the computer to determine the area under the peak curve. The area under the curve represents the number of protons that correspond to that resonance frequency. Therefore, integration is an INCREDIBLY USEFUL tool used to determine the number of each type of proton in a sample. You should ALWAYS consider integration before determining the structure represented by a spectrum.
The integral values are often given to you above or below each peak. In a real spectrum these values are generated electronically and represented by a curved line over the peak area.
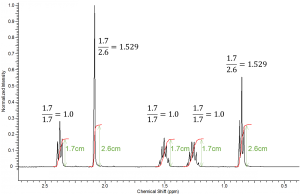
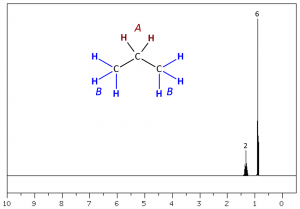
Integration values are relative. This means a spectrum of n-propane (above) could be integrated in several different ways. The two terminal methyl (CH3) groups (protons labeled B) are chemically equivalent showing one triplet peak with an absolute integration of six; whereas, the internal methylene (CH2) group (protons labeled A) would appear as a quadruplet with absolute integration of two. Since integration values are relative we could designate the integration values of CH3:CH2 as 6:2, 3:1, 1.5:0.5, 4:1.333, 24:8, etc. The relative relationship will be consistent but the numerical values do not necessarily represent the true number of methyl and methylene protons contained within the molecule.
To this point we have only discussed spectra that contain a single compound. Integration can also be used to determine the relative amount of each substance in a mixture. The method used is explained on the “determining amounts in a mixture” page. You should review chemical shifts and multiplicity analysis prior to attempting this type of problem.