More time with our beta design would have allowed us to commission more metal components to the drill guide. Most of the components we have are 3D printed, which is suitable for our testing purposes, but if this product were to go to market it would need to be optimized for stiffness, biocompatibility, and cost. Evaluating tolerances and ease of use with final materials would grant an extra level of credibility to our design. We would also be able to work to make the changes necessary found from the second phase of testing. Reworking the T tube design and completing Mirage in aluminum would significantly improve the device.
Another point of discussion that was brought up during the design review was to elongate the back end of the T tube, the add-on part that guides the drill for the ALL tunnel (see image below). Elongating the T-tube would offer greater support and stability for the drill, and therefore, result in a more consistent result. With more time, we would be able to test different lengths of T-tube in order to determine which length offers the greatest amount of accuracy and consistency. For this reason, we had to remove the guide between the drilling of the two tunnels.
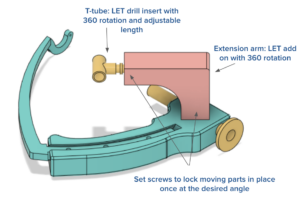
Due to testing, design, and time restraints, our guide had to be removed between the drilling of the ACLR tunnel and the ALLR tunnel. This was mentioned in the design review as something to be wary of, but it is important to note that the guide was removed between drillings largely for the purpose of ensuring ACL tunnel accuracy. Ultimately, this guide aims to allow surgeons to drill the ACLR tunnel , drill the ALLR tunnel, remove the guide, and then graft for both procedures. Ideally, we would have been able to obtain an Arthrex Flip Cutter III in order to drill an accurate and consistent ACLR tunnel rather than designing our own duplicate of this device, which required its own testing.