Objectives and Constraints
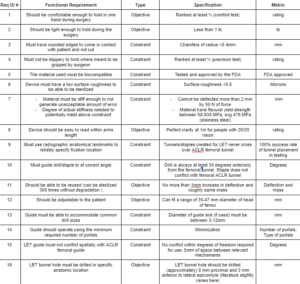
- This functional requirement will be tested with the use of a ranking system. The test will be performed by having each team member hold the instrument in the way it is intended for 3 minutes. Each team member will rank how comfortable the tool is to hold from 1-5, with 5 being very comfortable. We decided that in order for our device to pass this requirement, the tool should rank an average of at least ⅘.
- This requirement is based on the OSHA guidelines [1], which says a precision tool that is held at the angle this tool will be used at, should weigh less than 1 lb. We will test this using a weight scale.
- In other applications, for example at the company ASML, there is a general rule that for a product to be safe to handle without causing cuts, all edges must be chamfered to at least 0.4mm. (Industry Standard) We decided to follow this reasonable constraint for now and see if we need to chamfered it more later.
- If necessary in order to ensure a guide doesn’t slip while making measurements, have standard weight/force pulling on a set shape of material while being held with gloves and make sure it does not slip from grasp. This will have each team member hold up the set testing shape while it is being pulled by a weight, and each will complete certain motions that could potentially induce slipping. The tests will be compared with different surfaces to hold on to, and the testing will have a control surface and a number of different grips in order to compare relatively. Base again on team test ranking system from requirement (1) (ranking ⅘)
- Biocompatibility requirements of a material are outlined by the FDA. Because our device will be making contact with tissues, it has to be biocompatible and approved by the FDA [2].
- A medical device must be able to be sterilized. In order to make a surface cleanable to microbial level, a surface roughness (Ra value or average of deviation of the trace from the center line) of less than 0.8 micrometers is recommended by the EHEDG [3].
- As a guide or measuring device being handled by surgeons, it is important the guide does not deflect in a substantial way when being handled. Given the size of the tunnel the guide is directing is ~8mm, material deflection needs to be tested. The rig shouldn’t deflect more than 2mm when subjected to force comparable to what a human hand could make on the rig, around 50N. If in testing the drill puts significantly more force on the rig or deflection needs to be smaller, this can be updated [4].
- Since this device will have numbers printed on it, the numbers must be able to be read at 1 m away perfectly by someone with 20/20 vision. This requirement will be met with a test run by the team members. The device will be held 1 m away from the team member. We will each have to be able to read the numbers on the device correctly assuming we all have 20/20 vision. If every team member is able to read the numbers, the requirement is met.
- The first requirement to avoid tunnel conflict is drill insertion location. Since the Lemaire LET procedure is non-anatomical, the precise location of the exterior hole is not critical and the dominating anatomical reference is the location of the femoral ACLR tunnel [5]. Since the exterior of the femoral ACLR tunnel is visible to the operating surgeon, the interior hole is the only point that must be determined via anatomical landmarks in order to have complete information about the location of the femoral ACLR tunnel. Relevant radiographic anatomic landmarks [6] [7] [8] must be used to determine the location of the interior femoral ALCR tunnel opening within 2 mm of precision in the sagittal plane.
- The second requirement to avoid tunnel conflict is anterior drill angle. A 2021 study by Zhu et al. found that when the drill angle was directed at least 30 degrees anteriorly from the point of insertion, tunnel conflict with the femoral ACLR tunnel did not occur in any of twelve cadaveric knees [9]. The authors note that this angle may change with regards to patient size, race, or other factors, and thus we have decided our tool should be able to specify a range of 30-50 degrees anteriorly. In an interview, Dr. John Grant suggested that rotational play (present in multiple axes) when using the staple technique can cause tunnel conflict [12]. We must eliminate this possibility for tunnel conflict due to multiaxial laxity if we choose to address the staple method.
- Paying attention to the environmental and cost concerns, our device will not be single use. To ensure that the device can be used multiple times, our team has required that the device can be sterilized at least 300 times without any damage to the device. To test this our team will sterilize the device 10 times and measure any deflection or mass loss from each sterilization.
- The tool needs to be able to cover a range of leg sizes. Research has shown that the average diameter of the head of the femur for males is 44.37 mm and for females is 38.44 mm [10]. The tool will then work in a range of 35 – 47 mm to take into account that these are averages and that people may be outside of this range.
- Since our device may integrate both targeting and angle of the drill location as well as being the guide itself, it must be able to accommodate a small range of drill bit diameters to be used. In our interview with Dr. John Grant he noted that the tunnels for LET and ACLR vary in diameter, so it is important to ensure that our guide is capable of letting different size drill bits or other instruments through [12].
- To ensure minimum damage is done to the patient, reduce the chance of infection, and encourage rapid recovery times, it is important that the surgery be performed as minimally invasively as possible. In an interview, Dr. John Grant suggested that aside from the portal required for femoral ACLR, one additional portal will likely be required for the Lemaire LET tunnel or staple placement and IT graft harvesting [12]. The portals should be as few and as small as possible without reducing function [11].
- This functional requirement becomes especially relevant if our final design combines femoral ACLR and Lemaire LET guides into one piece of equipment. As specified during our interview with Dr. John Grant, the drill guide will likely need to have two independent arms for ACLR and LET; if this is the case, they must not hinder each other during use. This includes conflict of guide arms as well as conflict of drill tunnel shafts [12]. To be certain, the mechanisms should maintain a tolerance of 5mm from each other.
- It is important to specify the location of the entry point on the harvested end of the IT band for the LET add-on. Literature slightly varies with the measurements specified (by Dr. John Grant), and since these measurements are not strictly anatomical, these may not serve as the final metric by which we ultimately choose LET tunnel drill location, but they are a good starting point.
[1] https://www.ccohs.ca/oshanswers/ergonomics/handtools/tooldesign.html
[3] https://www.safefoodfactory.com/en/editorials/70-roughness-measurements-stainless-steel-surfaces/
[4]https://www.matweb.com/search/DataSheet.aspx?MatGUID=71396e57ff5940b791ece120e4d563e0&ckck=1
[5] Interview with Dr. Ben Saks
[7] For sMCL: Radiographic landmarks for locating the femoral origin of the superficial medial collateral ligament (Hartshorn et al. 2013)
[8] For PCL: Radiographic Landmarks for Tunnel Positioning in Posterior Cruciate Ligament Reconstructions (Johannsen et al. 2013)
[9] Zhu et al. https://www.webofscience.com/wos/woscc/full-record/WOS:000617153500001
[11] https://www.orthobullets.com/approaches/3031/knee-arthroscopy
[12] Interview with Dr. John Grant (add link once in website)