Micro Fluidic Point-Of-Care Diagnostics
Group Members:
Tom Pregel, Mike Kaplan, Jake Levy
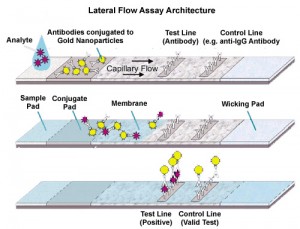
On today’s edition of nano tech, our host Mike talks about Micro Fluidic Point-of-Care devices and interviews a local CVS representative. A POC device stands for Point-of-Care device, like a pregnancy test, that does not require the need of a laboratory or medical experience. In order to make these devices one must have the knowledge of many different fields and enough resources to cover the expense of creating them. To engineer a POC device one must know about the reaction that they are trying to make/ what they are looking for in order to design the microfluidic chip.
Great podcast! Clearly engineering has quite a few hurdles to overcome as its principle products are integrated into society. There are many arguments against the usage of private diagnostic devices, but I think the pros will eventually outweigh the cons. Accessibility is also another topic of discussion because as you pointed out cost is a major factor. Is funding/money ultimately what is comes down to?
Very interesting topic. I like how you included the point of view of a CVS representative to give another opinion on this controversial subject. Very good job covering all the bases on the pros and cons of this issue. I believe that POC devices will help those at a disadvantage, such as having no health insurance to be able to visit a doctor or not being able to afford a pricey device, diagnose themselves and therefore create higher awareness and decrease the spread of HIV. But I am glad I was able to listen to all the different opinions on this subject.
Great podcast, I was not aware that POC HIV diagnostics were open to the public yet. It is concerning that POC diagnostics could prevent the CDC from tracking the spread of HIV. In my opinion, any technology that could potentially lead to an outbreak of HIV infections should be implemented extremely carefully.
It’s just like the type of test that Prof. Ferri was talking about in class. If they could create these for types of cancer it would be earth-shattering. I can’t believe they have poc units for HIV. If the public cared less about the negative stigma for HIV we would already have cheaper and easier private tests.
Nicely done. I was unaware that diagnostics for HIV through POC’s were available. Obviously, while it is an incredible technology, it is not very well known. The cons and pros of private testing were described through every perspective. Well rounded.
I enjoyed listening to your podcast presentation, and it was good how you highlighted that the idea of shifting to at home HIV testing is still highly controversial. I am concerned that if these tests do become easily excusable to the public, cost effective, and approved by the FDA, it could open Pandora’s box to the spread of HIV. Once the general public has the choice of what to do with their diagnostics, it could lead to an epidemic that might be difficult to regain control over. Do you personally believe that these tests could be effectively implemented into a developing country or a developed country more effectively?