Nuclear fission is the process by which heavy elements split to form lighter elements, giving off energy. Fundamentally, fission is a exothermic reaction, capable of releasing large amounts of energy in the form of both electromagnetic radiation and kinetic energy. Nuclear fission is an extremely efficient process, as it gives off 108 times as much energy per atom compared to the burning of fossil fuels.[1]
In engineered nuclear devices, almost all nuclear fission takes place as a nuclear reaction- a bombardment driven process that results from the collision of a subatomic particle and an atomic nucleus. The subatomic particle (in most cases a slow-moving neutron) collides with a nucleus, causing the nucleus to split into two smaller particles. While nuclear fission can occur without the neutron bombardment, in processes known as spontaneous fission or radioactive decay, nuclear reactors utilize the more controlled, bombardment fission reactions.
Fuel
The most common nuclear fuels are Uranium-235 and Plutonium-239.While Uranium is relatively abundant on Earth, only two isotopes of Uranium are found in nature: U-235 and U-238. The difficulty with using Uranium as nuclear fuel come about because U-235 is the only isotope that is able to sustain a chain of fission reactions, i.e it is the only isotope that is fissile. However, only 0.7% of the world’s total mined Uranium resource is U-235. That means the remaining 99.3% of natural Uranium is U-238, which is not useful for nuclear reactors.[3]
Plutonium-239 has a half life of 24,110 years.[4]
Uranium-235 has a half life of 704 million years.[3]
When bombarded with neutrons, these heavy nuclei fission, splitting into two lighter fragments and emitting more neutrons. As illustrated by the image below, the resulting fragments, also called fission products, for a typical Uranium reaction consist of Barium-141, Krypton-92, and an average of three neutrons.[2]
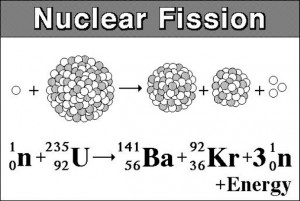
-
A common nuclear fission reaction, where a Uranium-235 nucleus is bombarded with a neutron particle. This causes the U-235 nucleus to split, producing, on average, Barium-141, Krypton-92, and three neutrons.
Nuclear Reactors
In a typical nuclear power plant, the fission process is just another way of producing heat. The heat produced by fission boils water to make steam used to drive a turbine, which is connected to an electric generator. The process of converting the heat energy given off by the fission reaction into electrical energy by means of the generator is essentially the same as a coal or natural-gas burning power plant.
Heat from nuclear fission is generated in the reactor core. The reactor core is loaded with fuel, which may be a combination of U-235 and U-238, although the chain reaction only occurs in the U-235. The reason behind can be understood by analyzing the relationship between the average kinetic energy of colliding neutrons and the probability of fission occurring, as depicted below. It is clear that very slow moving neutrons (kinetic energy equal to about 10-2 eV) have about a thousand times more probability of fissioning U-235 than do faster moving neutrons (about 106 eV) on either U-235 or U-238.[1]
This poses a problem for reactors, as neutrons coming directly from fission reactions have an average kinetic energy of about 2 MeV. Thus, the first step in a nuclear reactor must be to slow down the two or three fission neutrons. This is accomplished by using a moderator, typically water or graphite, to slow down the neutrons. Eventually, after some time spent bouncing around the moderator, the fission neutrons will have the same kinetic energy as the thermal energy (temperature) of the moderator, which is about 0.025 eV, slow enough for a high probability of U-235 fission.[2]
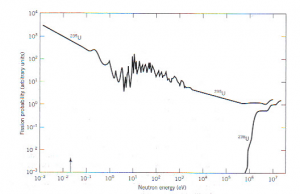
-
Fission probability for U-235 and U-238 as a function of neutron kinetic energy. For U-238, fission becomes probably only above about 1 MeV neutron kinetic energy.[1]
(Composed by Sean Hanczor, Edited by Dan Kervick)
References
[1] Ristinen, Robert A., and Jack J. Kraushaar. “The Promise and Problems of Nuclear Energy.” Energy and the Environment. 2nd ed. N.p.: John Wiley & Sons, 2006. 171-202. Print.
[2] “Physics of Uranium and Nuclear Energy.” World Nuclear Association, Sept. 2014. Web. 29 Apr. 2015.
[3] “Uranium: Its Uses and Hazards.” Institute for Energy and Environmental Research. N.p., May 2012. Web. 30 Apr. 2015.
[4] “Physical, Nuclear, and Chemical Properties of Plutonium.” Institute for Energy and Environmental Research. N.p., Apr. 2012. Web. 06 May 2015.
