Answers to Questions
Ethyl bromoacetate splitting
 |
Resonance A will appear as a singlet (no neighbors). Resonance B will be a quartet due to the neighboring methyl protons. Finally, the peak corresponding to methyl group C will appear as a triplet due to coupling with methylene group B. |
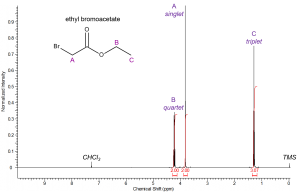 |
Your sketch should appear similar to the actual spectrum of ethyl bromoacetate to the left. Resonance A will be a singlet integrating to 2 and will appear downfield relative to most methylene protons due to the electron withdrawing effect of bromine. Resonance B is adjacent to an oxygen atom and is therefore expected to appear around 4ppm, the ester functionality is expected to deshield the protons slightly more than an ether group. Resonance B appears as a quartet due to coupling with methyl group C and will integrate to 2 protons. Lastly, resonance C will be upfield of all the other peaks due to its proximity from any electronegative functionalities. It will integrate to 3 protons and appear as a triplet due to spin-spin splitting with methylene B. |